Position papers

AIM recommendations to the EC Roadmap on Food labelling
AIM sees potential in the Roadmap of the EC on the Revision of Informa...
05.02.2021- Read more

AIM answers the Consultation on the Pillar of Social Rights
AIM welcomes communication from the European Commission on “Building...
01.12.2020- Read more

Tackling Medical Deserts across the EU
In the context of the European Commission Consultation on a "Long Term...
30.11.2020- Read more
COVID-19: AIM publishes Recommendations for Europe & Regional Declarations
Today, AIM’s Board of Directors and General Assembly of has adopted...
18.11.2020- Read more

Joint Statement – Transparency is needed to reap the full benefits of the EU’s investment in its Vaccines Strategy
Ahead of Friday 18 September's webinar the EU Vaccine Strategy, a numb...
21.09.2020- Read more
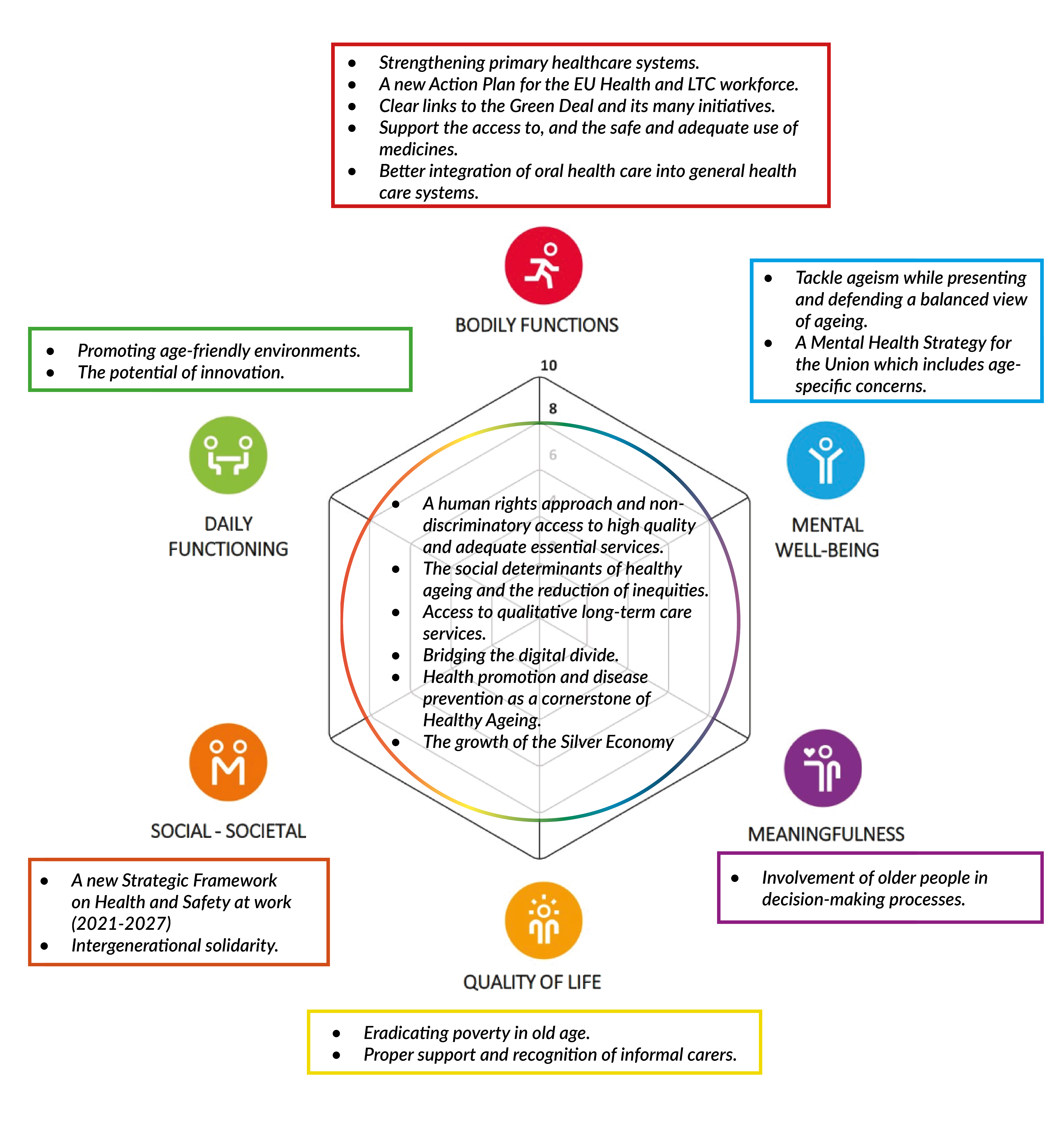
AIM Recommendations on the Green Paper on Ageing
AIM welcomes the European Commission’s intention to publish a Green...
06.07.2020- Read more
